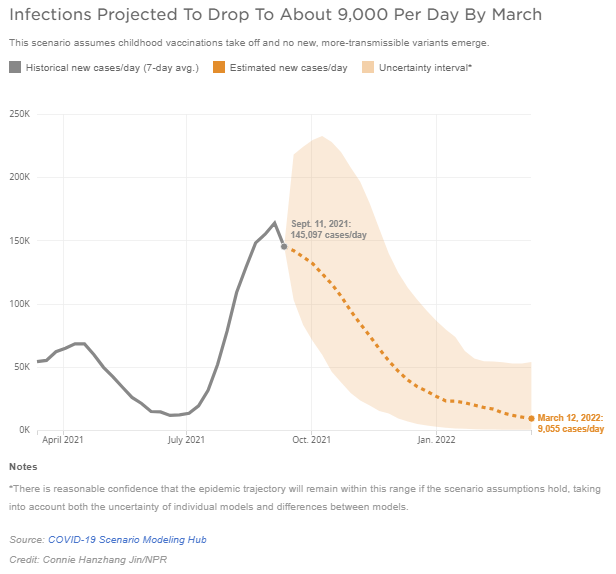
Boston – According to a new analysis by a consortium of researchers advising the CDC, the Delta surge will likely decline steadily now through next spring — without a significant bump up in the winter. As a result, we expect this will be our last weekly update on health policy responses and other COVID developments.
Health Policy Responses
- An FDA advisory panel voted on September 17 against approving a booster shot for people 16 and older who received the Pfizer/BioNTech vaccine. On September 22, however, the FDA did approve a third dose for anyone 65 or older and those aged 18 to 64 who are vulnerable to severe COVID or working in jobs with a high risk of exposure.
- The U.S. will require adult travelers to be vaccinated for entry into the country.
- Unvaccinated heads of state attending the United Nation General Assembly this week will not be allowed to eat at New York’s restaurants.
- Tennessee is limiting monoclonal antibody treatment to people who are not vaccinated, and Idaho allowed overwhelmed hospitals to ration care if necessary.
- Canada gave full approval to the Pfizer/BioNTech and Moderna vaccines and authorized new names of Comirnaty and Spikevax, respectively, while the AstraZeneca vaccine will be called Vaxzevria; 69% of all Canadians are fully vaccinated.
- In Asia, Cambodia began vaccinating 6-to-11 year olds, mostly with China’s Sinovac and Sinopharm; Vietnam has given emergency-use approval for Cuba’s Abdala COVID-19 vaccine; and Singapore’s prime minister has received a third dose of the Pfizer vaccine, eight months after his initial dose.
- In Europe, around 3,000 French health care workers have been suspended for refusing to be vaccinated, and the Pope urged everyone to get a COVID-19 vaccine.
Bullish Virus Developments
- New cases in the U.S. have clearly rolled over, despite the return to school.
- Vaccinated pregnant women pass protection to babies, according to a new study in the American Journal of Obstetrics & Gynecology, but only 30% of pregnant women are vaccinated.
- People given a booster shot six months or longer after their first dose had a 12-fold increase in antibodies — compared to a four-fold increase for those who got a second dose at two months. This study suggests protection would be stronger if boosters were received later.
- A two-dose version of the Johnson & Johnson vaccine is 94% effective against COVID-19.
- Blood tests on 8,500 individuals were used to estimate that almost 90% of people in Mumbai — India’s financial capital with a population of 12.5 million — have COVID-19 antibodies.
Bearish Virus Developments
- A new CDC study has found that during a COVID-19 outbreak involving the Delta variant in a highly vaccinated incarcerated population, transmission rates were high, even among vaccinated persons. The conclusion is that congregate settings are a particular challenge even for the vaccinated.
- A CDC study released last week showed that 18% of COVID cases, 14% of hospitalizations and 16% of deaths are now occurring in fully vaccinated people — a substantial increase from the 5% to 8% infection, hospitalization and death rates seen just two months before.
- Moderna’s vaccine protection wanes by 36% after 12 months according to a new study, making the case for a booster shot.
- One in three unvaccinated COVID-19 patients suffers from long COVID, with fatigue the most commonly reported symptom.
- There is a shortage of rapid COVID-19 antigen tests.
Other Developments
- Pfizer versus Moderna, according to Dr. Jeffrey Wilson, an immunologist at the University of Virginia: “Pfizer is a big hammer. Moderna is a sledgehammer.”
- Zoom is offering booths that allow workers to keep zooming when they return to the office.
- PhDComics.com has received high praise by scientists for explaining SARS-CoV-2. One fun fact: Before 2020, there were 700 scientific papers per year on coronaviruses; that annual figure now tops 70,000.
Source of all data: Eaton Vance research as of September 23, 2021 unless otherwise specified.

