The cost of successfully bringing a new drug to market has never been higher.
Research has shown that the average cost amounts to $1.3 billion USD¹. The process is also lengthy, often taking more than ten years to bring a research lab discovery to Food & Drug Administration (FDA) approval, and eventually into patients’ hands. Yet, only 10% of the drugs that start pre-clinical trials ever make it to the end.2 According to a research done by Deloitte, return on new drug research & development (R&D) has fallen from 10.1% in 2010 to 1.9% in 2019.3 FDA approved 53 novel drugs in 2020, the second highest count in over 20 years, contrasting with over 123,000 trials currently ongoing in the US.4
Even after a successful launch, getting the right drug into the hands of the right patient is not as straightforward as one might think, often complicated by the patient’s insurance coverage (or the lack of), co-pay program, the doctor’s awareness of the drug, etc. Pharma representatives, whose mission is to educate doctors about novel drugs, spend most of their time travelling and trying to catch doctors face-to-face in between their patient appointments. These meetings last on average only three minutes, just allowing sales reps to drop off samples and a few printed paper brochures.5 How to be effective in these three minutes is a big challenge.
This [connecting sites, patients and sponsors using Veeva Clinical Network] is a bold vision for the industry that over time has the potential to make [clinical] trials 25% faster at 25% less cost.
Peter Gassner, co-founder and CEO, Veeva Systems, March 2021 (fiscal 4Q21 earnings call)
Then medication non-adherence has persisted as a serious problem as it affects treatment success, leads to worsening of disease, and increased healthcare cost. Some call it “the silent killer”. Extensive research has shown that even in developed countries, adherence to therapies averages only 50%, whereas half of the non-adherences being intentional.6 Adherence rates are typically lower among chronic disease patients compared to patients with acute conditions.
We are seeing innovation accelerating across the different stages of drug development (see picture 1). AI and machine learning have just started to show their potential as powerful tools and possibilities seem to be endless. Some of them might transform biology and healthcare for good.
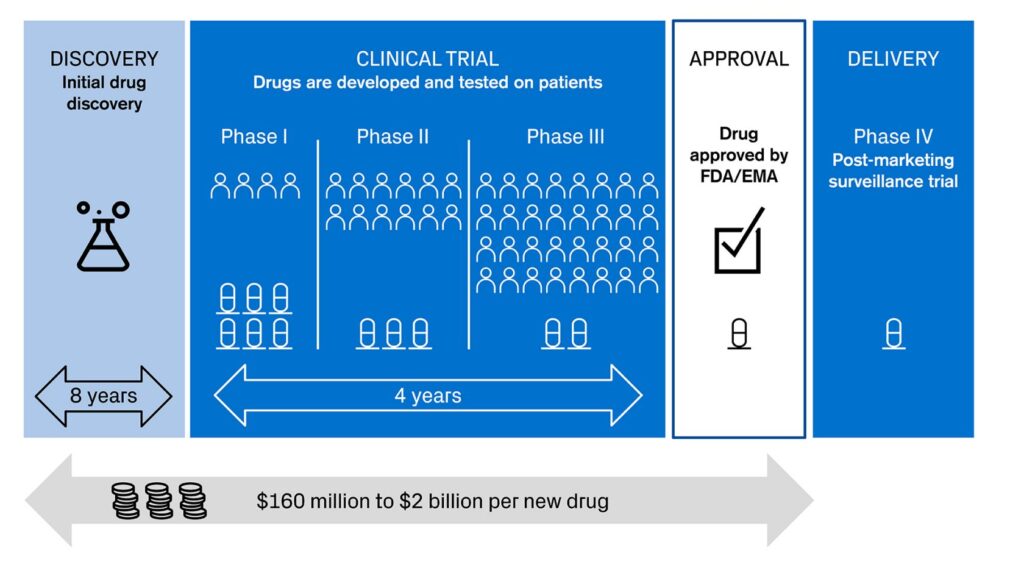
Picture 1: Different stages in drug development
Sources: Credit Suisse, based on KEYRUS | 4 ways Digital Health is helping big pharma in modernizing clinical trials
Computational R&D redefining drug discovery
Traditional drug discovery process relies on many reiterations of trial-and-error. It is labor intensive, inefficient, slow, and prone to high failure rates. It often takes years and millions of dollars just to identify a target and validate a “hit compound” – a molecule that shows the desired activity in a screening assay, which could potentially be turned into a drug.
Computational R&D companies look to improve this process by offering simulation tools that allow scientists to predict drug properties and design new drugs using models, the same way that virtual plane engines are designed and simulated before being prototyped on the factory floor. This could greatly reduce the time it takes to develop the drug and at the same time improve the quality of the final drug.
One example was the gigantic leap of Alphabet DeepMind’s AlphaFold, which uses deep learning to predict the 3D structure of proteins at high accuracy.7 Some believe it will transform biology. Google’s AI has also shown great potential of outperforming radiologists in lung cancer and mammographic screening based on CT scanner images.8
Last year, together with partner Eli Lilly, Abcellera, a next-generation therapeutic antibody discovery company, brought to life the first antibody treatment Bamlanivimab, developed for COVID-19 pandemic in only nine months.9 Abcellera screens the diversity of the human immune system for antibody discovery, incorporating microfluids, single-cell analysis, high-throughput genomics, machine learning and hyper-scale data sciences. It took Abcellera only 90 days from receiving the COVID-19 antibody sample from a recovered patient to the phase of human testing. Bamlanivimab received FDA emergency use authorization in November 2020.
Another company Recursion Pharmaceuticals utilizes machine learning, big data computational tools, lab robotics and databases to analyze potential chemical interactions of derived images of proteins. It aims to broaden the breadth of potential starting points and identify failures earlier in the drug discovery process.
Pressure to eliminate animal testing is another driver of using computational simulation. In 2019, the US Environmental Protection Agency (EPA) committed itself to reduce its funding request for animal studies by 30% by 2025 and phase them out entirely by 2035.10
Leaner and less costly clinical trials
Clinical trials typically consume half of the entire drug development cost, whose total spend often adds up to over $1bn as mentioned above. Figure 1 shows the rough breakdown of various costs:
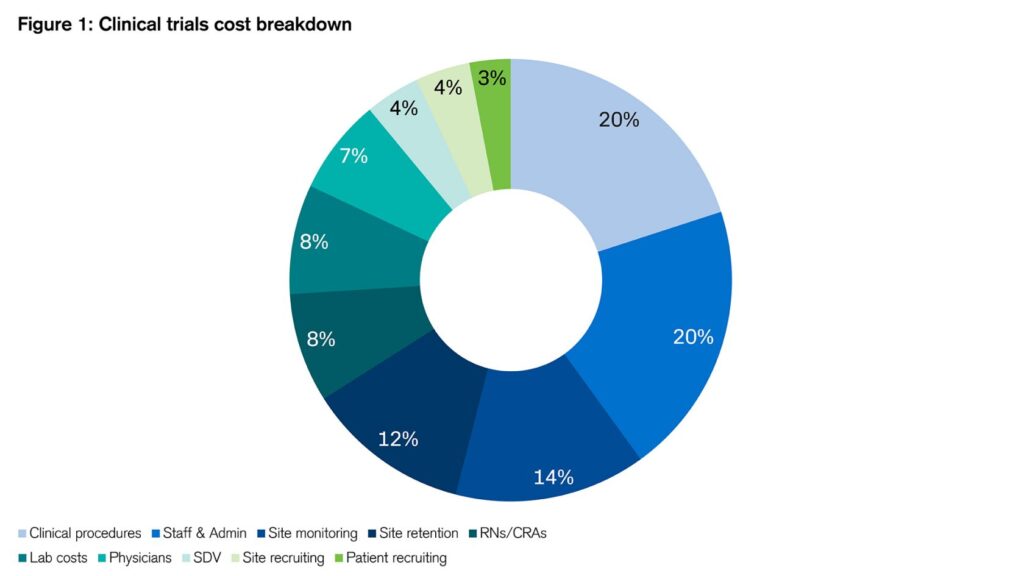
Figure 1: Clinical trials cost breakdown
Sources: Credit Suisse, Clinicalresearch.io¹¹
Figure 1: Clinical trials cost breakdown
Sources: Credit Suisse, Clinicalresearch.io¹¹
Among the causes of trial failures, most cited reasons include failure to recruit enough eligible patients, mid-trial patient drop out, side effects and inconsistent data. Patient recruitment is also the most common reason of trial delay with nearly 80% of the trials failing to finish on time due to lack of enrolees. A study showed that only 3% of the cancer patients in the US are enrolled in clinical trials.12
On the other side, potentially eligible patients struggle to find the trial that suits them. Using online marketing as an analogy, there is no such thing as “personalized targeting” that allows the advertiser to target precisely the relevant consumers. Typically, a patient’s journey starts with browsing on clinicaltrial.gov which records all the currently ongoing clinical trials in the US. At any time, more than 20,000 clinical studies are recruiting patients. Occasionally, the patient’s physician can recommend a trial provided that he is aware of it.
Then the patient often needs to take on the burden of trial application himself. There is no centralized repository of patient medical data. Clinical data is often scattered across different doctors the patient has visited, which is stored on different electronic health record (EHR) systems with no interoperability between them. Some documents are sent by fax or scanned hand-written copies which are prone to error. It often takes the patient a substantial amount of time and effort to gather all the clinical information needed to apply for the trial and yet, only 30% would meet the enrolment criteria.13 Under the Health Insurance Portability and Accountability Act (HIPAA), data sharing is now allowed with patient’s authorization, but the lack of system interoperability and related data security issues still makes it a cumbersome process for patients.
Once the patient is enrolled in the trial, it can last as long as six to seven years. Patients are asked to keep their physiological data diary, which is still often paper-based, prone to human error, and overly reliant on patient memory. Then patients need to travel to the participating site regularly and share these data with site researchers with a various degree of time delay. This burdensome and inconvenient process leads to an average of 30% patient dropout rate.14
The need of remote patient monitoring is even more pronounced in novel technologies such as gene and cell therapies. These clinical trials need less patients, often only a dozen, but will need longer follow-up period, which offers a good use case for remote monitoring devices as a means to reduce burden on patients.
Veeva Systems is a software company developing applications for life science industry. It offers a suite of applications that span the full drug development lifecycle. Its Vault platform unifies clinical, regulatory, quality and safety functions in drug development process. As CEO Peter Gassner shared on its Investor day in 2020:
We have a full vision to change the way trials are executed. We want to make clinical trials paperless and patient-centric.
Peter Gassner, co-founder and CEO, Veeva Systems (October 31, 2020, Veeva Systems Investor day)
As a result, we believe significant opportunities are untackled in optimizing trial design, patient recruiting, electronic data collection, regulatory approval and patient adherence using technologies such as big data analysis, AI, remote device monitoring, which ultimately would help clinical trials to recruit the right patient at lower cost and in the mean time offer a more patient-friendly experience.
Drug commercialization moving online
A study has found that almost 20% of big pharma company revenues go to marketing and advertising – this represents a total spending of $50bn just for the ten largest drug makers in the U.S.15 A substantial portion of the spending goes to the pharma sales reps, whose mission is to educate the healthcare professionals about the drugs. There are 81,000 pharma sales reps in the US reaching out to about 830,000 doctors.16
Despite tools such as CRM (Customer Relationship Management) that allows life science sales reps to manage, track and optimize their interactions with healthcare professionals, offline face-to-face visits remain the primary form of contact as of today. The COVID-19 pandemic democratized the adoption of virtual visits, as people were confined at home. Interestingly, doctors have shown high satisfaction towards the virtual format, along with increased productivity of sales reps. Veeva found that virtual meetings last between 10 and 40 minutes, significantly higher than the three minutes face-to-face ones.17 Some speculate that the increase in productivity will allow pharma companies to reduce sales reps headcount and that digital marketing will continue to grow after the pandemic.
In a survey conducted by M3 – a leading doctor network operator in Japan – in July 2020, “75% of physicians prefer current condition of higher [more extensive] digital information procurement”, which prompted M3 to think that this shift in behavior and sentiment is likely to remain.18
Novel solutions for patient adherence
Studies have shown that 20-30% of drug prescriptions are never filled in the US, and that approximately 50 percent of medications for chronic disease are not taken as prescribed. This lack of adherence, is estimated to “cause approximately 125,000 deaths and at least 10 percent of hospitalizations, and to cost the American health care system between $100 billion and $289 billion a year.” 19
Fulfilment non-adherence is often attributed to lack of insurance coverage and high deductibles or high co-pay. Other factors of non-adherence include non-persistence and non-conformation. Some are intentional while some others are not. But in either case, better communication between the physician and the patient and using technology to facilitate the process would prove to be beneficiary.
Some companies such as OptimizeRx are looking for innovative ways to address this issue, trying to reach out to potential patients directly, improve patient drug adherence and build their own database. This could be particularly valuable for other stakeholders such as FDA and pharma companies who are increasingly interested in RWE (Real World Evidence) as a supplementary of RCT (Randomized Controlled Trials)20.
Can you imagine spending billions of dollars on developing a drug and not being able to communicate or educate [physicians and patients] … on its clinical benefit, cost, and adherence?
Will Febbo, CEO OptimizeRx (Q3 2018 earnings call)
Conclusion
About 5% of the world’s data are generated by the healthcare industry in the year of 2020.21 Yet, they are largely underutilized to generate benefits for all. In this article, we discussed how the entire lifecycle of a drug can become more digital, from drug discovery, clinical trials, regulatory approval, to commercialization and patient adherence etc. Along with it, with customer compliance, data collection will become more accurate, timely, consistent and systemic.
In such a scenario, there is tremendous benefit of having an integrated cloud platform encompassing software solutions, applications, data warehouses, AI and analytics which facilitates seamless data and information flows between the different end points so as to generate the most actional insights and agile decision makings for all the stakeholders.
It is safe to argue that we are at the cusp of a generational leap forward in novel therapies development in medicine, thanks to which disease treatment will be transformed and incurable diseases will become treatable or preventable. Digital tools and technologies will play a critical role as we embrace this new world.
Credit Suisse Asset Management has designed a number of highly focused strategies to provide clients with “pure-play” exposure to a number of compelling long-term secular growth themes, such as Robotics & Automation, Security & Safety, Digital Health, Edutainment and Environmental Impact.
The individuals mentioned above only conduct regulated activities in the jurisdiction(s) where they are properly licensed, where relevant.
The securities mentioned on this page are meant for illustration purposes only and are not intended as a solicitation or an offer to buy or sell these securities.
To the extent that these materials contain statements about the future, such statements are forward looking and are subject to a number of risks and uncertainties and are not a guarantee of future results.
1 Wouters OJ, McKee M, Luyten J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018. JAMA. 2020;323(9):844–853. doi:10.1001/jama.2020.1166
2 Modern Drug Discovery: Why is the drug development pipeline full of expensive failures? – Science in the News (harvard.edu), accessed on May 13, 2021
3 Measuring the Return from Pharmaceutical Innovation 2019 | Deloitte US, accessed on May 13, 2021
4 https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2020, Trends, Charts, and Maps – ClinicalTrials.gov, both accessed May 14, 2021
5 ZS Associates’ Spring 2014 AccessMonitorTM Survey, https://www.zs.com/insights, accessed May 14, 2021
6 Patient Medication Adherence: Measures in Daily Practice (nih.gov), accessed May 14, 2021
7 AlphaFold: a solution to a 50-year-old grand challenge in biology | DeepMind, accessed May 14, 2021
8 AI outperforms radiologists in mammographic screening | Nature Reviews Clinical Oncology, accessed May 14, 2021
9 AbCellera-Discovered Antibody, Bamlanivimab, Administered with Etesevimab Receives FDA Emergency Use Authorization for COVID-19, accessed May 14, 2021
10 EPA Finalizes Guidance to Waive Toxicity Tests on Animal Skin | U.S. EPA News Releases | US EPA, accessed May 14, 2021
11 Cost of Clinical Trials: A Breakdown (Infographic) – Clinical Research IO – CRIO, accessed May 14, 2021, data as of 2016
12 M.N. Fouad, J.Y. Lee, P.J. Catalano, T.M. Vogt, S.Y. Zafar, D.W. West, C. Simon, C.E. Klabunde, K.L. Kahn, J.C. Weeks, C.I. Kiefe Enrolment of patients with lung and colorectal cancers onto clinical trials J. Oncol. Pract., 9 (2) (2013), pp. e40-47;
13 P. Bower, P. Wallace, E. Ward, J. Graffy, J. Miller, B. Delany, A.L. Kinmonth Improving recruitment to health research in primary care Fam. Pract., 26 (2009), pp. 391-397, 10.1093/fampra/cmp037
14 National Academy of Sciences. The Prevention and Treatment of Missing Data in Clinical Trials. Washington, D.C.: National Academies Press; 2010. Available at: www.nap.org.
15 The Big Pharma Dollar: Investing Boldly in Advertising & Profits – NOT R&D. – CSRxP, accessed May 14, 2021
16 ZS Associaee, Pharmaceuticals. ZS: Global professional services firm | Analytics, technology, strategy
17 Veeva Investor day, October 30, 2020
18 M3 Inc, Q2 2020 earnings presentation, page 12.
19 Viswanathan M, Golin CE, Jones CD, Ashok M, Blalock SJ, Wines RC, Coker-Schwimmer EJ, Rosen DL, Sista P, Lohr KN. Interventions to improve adherence to self-administered medications for chronic diseases in the United States: a systematic review. Ann Intern Med. 2012 Dec 4;157(11):785-95. doi: 10.7326/0003-4819-157-11-201212040-00538. PMID: 22964778.
20 RWE data is generated from a variety of sources, such as EHR (Electronic Health Record), medical claims, wearables, biosensors, or other modalities, and is used to help regulators and pharma companies better assess postmarket drug safety.
21 Healthcare data volume globally 2020 forecast | Statista, How Much Data Is Created Every Day? [27 Powerful Stats] (seedscientific.com), accessed May 14, 2020
This material constitutes marketing material of Credit Suisse Group AG and/or its affiliates (hereafter “CS”).
This material does not constitute or form part of an offer or invitation to issue or sell, or of a solicitation of an offer to subscribe or buy, any securities or other financial instruments, or enter into any other financial transaction, nor does it constitute an inducement or incitement to participate in any product, offering or investment.
This material does not constitute investment research or investment advice and may not be relied upon. It is not tailored to your individual circumstances, or otherwise constitutes a personal recommendation.
The information may change after the date of this material without notice and CS has no obligation to update the information.
This material may contain information that is licensed and/or protected under intellectual property rights of the licensors and property right holders. Nothing in this material shall be construed to impose any liability on the licensors or property right holders. Unauthorized copying of the information of the licensors or property right holders is strictly prohibited.
This material may not be forwarded or distributed to any other person and may not be reproduced. Any forwarding, distribution or reproduction is unauthorized and may result in a violation of the U.S. Securities Act of 1933, as amended (the “Securities Act”). The securities referred to herein have not been, and will not be, registered under the Securities Act, or the securities laws of any states of the United States and, subject to certain exceptions, the securities may not be offered, pledged, sold or otherwise transferred within the United States or to, or for the benefit or account of, U.S. persons.
The only legally binding terms of any investment product described in this material, including risk considerations, objectives, charges and expenses are set forth in the prospectus, offering memorandum, subscription documents, fund contract and/or any other fund governing documents.
Prospective investors should independently and carefully assess (with their tax, legal and financial advisers) the specific risks described in such materials, and applicable legal, regulatory, credit, tax and accounting consequences prior to making any investment decision.

